Decoding the Facts: Clear Choice Failure Rate - What You Need to Know
In the ever-evolving landscape of dental care, making informed choices about dental implants is crucial for long-term success.

Knowing how to calculate theoretical yield can be helpful in the laboratory, but many students struggle with the concept. Let’s break down what you need to know about calculating academic work!
Buying stamps can be an overwhelming experience. There are many types of stamps, and prices range from reasonable to outrageous. And once you've finally purchased your stamps, there's the question of where to buy them so you won't have to deal with this hassle again. Here are some tips to help you make better decisions about how many stamps you need and where to get them when you need them next time!
Table of contents [Show]

The theoretical yield is the amount of money you can expect to make. It is the amount of money in sales you will be able to reinvest in your business based on its current expenditures. This number can be calculated using a simple formula:
sales/expenses = return on investment (ROI).
In other words, if your company earns $100K and spends $50K, your ROI is 50%. Now, let's say you are selling T-shirts for $30 each.
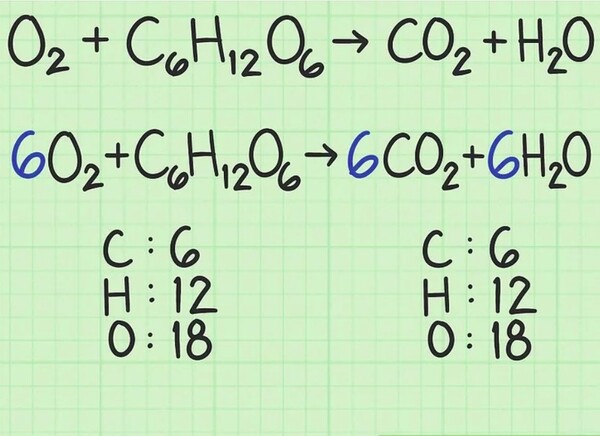
A chemical equation is a recipe for a theoretical yield. It suggests the reactants (at the left facet) react to shape products (at the proper aspect). A nicely balanced equation will display the identical wide variety of atoms going into the equation as reactants as you've got popping out withinside the shape of products.[1]
There are atoms of hydrogen on each leaf. But there are atoms of oxygen getting in as a reactant, and the handiest one bit withinside the product at the proper. For example, don't forget the easy equation +O_}H_+O_ → O}H_O.
To balance, double the product, to get +O_}H_+O_ → {displaystyle 2H_O}2H_O.
Check the balance. This extrude has corrected the oxygen, which has atoms on each side. But you presently have atoms of hydrogen at the left and four atoms of hydrogen at the right. Double the hydrogen within the reactant. This will alter the equation to {displaystyle 2H_+O_}2H_+O_ → {displaystyle 2H_O}2H_O. This extruded material now has four hydrogen atoms on each side and atoms of oxygen. The equation is balanced.
As an extra-complex example, oxygen and glucose can react to shape carbon dioxide and water: display style 6O_+C_H_O_6O_+C_H_O_ display style 6CO_+6H_O6CO_+6H_OIn this line, every facet has precisely six carbon (C) atoms, 12 hydrogens (H) atoms, and 18 oxygen (O) atoms. The equation is balanced.

I am using the periodic table or a few different references and adding up the molar mass of every atom in every compound. Add them collectively to discover the molar mass of every reactant mixture. Do this for an unmarried molecule of the mix.[2] Consider once more the equation of changing oxygen and glucose into carbon dioxide and water: +C_H_O_}6O_+C_H_{}O_ → {displaystyle 6CO_+6H_O}6CO_+6H_O
For this example, one molecule of oxygen ({displaystyle O_}O_) includes oxygen atoms.
The molar mass of 1 atom of oxygen is set to sixteen g/mol. If necessary, you may discover particular extra values.)
2 oxygen atoms x sixteen g/mol consistent with atom = 32 g/mol of {displaystyle O_}O_.
The different reactant, glucose ({displaystyle C_H_O_}C_H_{}O_) has a molar mass of (6 atoms C x 12 g C/mol) + (12 atoms H x 1 g H/mol) + (6 atoms O x sixteen g O/mol) = a hundred and eighty g/mol.
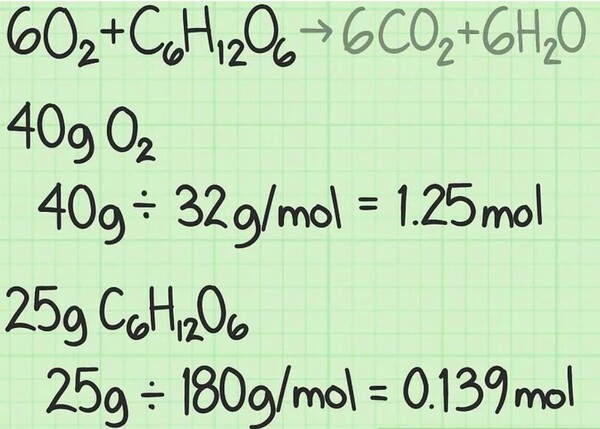
For a natural experiment, you'll recognize the mass in grams of every reactant used. Divide this amount using that compound's molar mass to transform the quantity into moles. [3]
For example, suppose you start with forty grams of oxygen and 25 grams of glucose: forty grams of O_ / (32 g/mol) = 1.25 moles of oxygen; 25 grams of H_O_C_H_O_ / (a hundred and eighty g/mol) = approximately 0.139 moles of glucose.
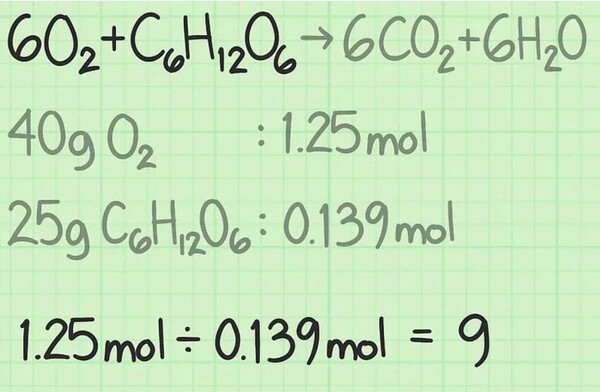
A mole is a chemical device to measure molecules' mass. By figuring out the wide variety of moles of each type of oxygen and glucose, you already know what number of molecules you're starting with. To discover the ratio between the two, divide the wide variety of moles of one reactant with the aid of the wide variety of moles of the other.
In this example of determining the molar ratio, you're beginning with 1.25 moles of oxygen and 0.139 moles of glucose. Thus, the percentage of oxygen to glucose molecules is 1.25/0.139 = 9.0. In this ratio method, you have nine instances of as many molecules of oxygen as you've got of glucose.
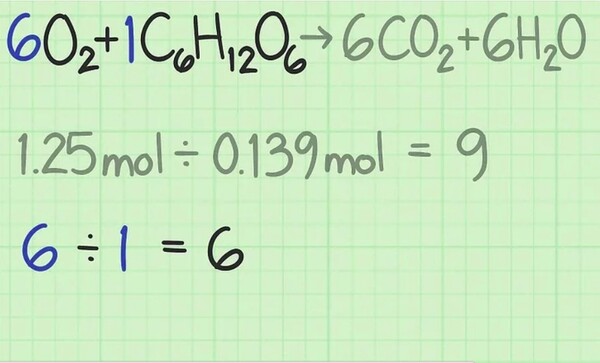
Look at the balanced equation for the response. The coefficients on the front of every molecule let you know the number of molecules you want for the Reaction to occur. If you operate precisely in the balance given with the aid of the formula, then each reactant has to be used equally. [5]
For this response, the reactants are given as +C_H_O_6O_+C_H_O_. The coefficients suggest that you want six oxygen molecules for each glucose molecule. The best ratio for the Reaction for this response is six oxygen to 1 glucose, or 6.0.
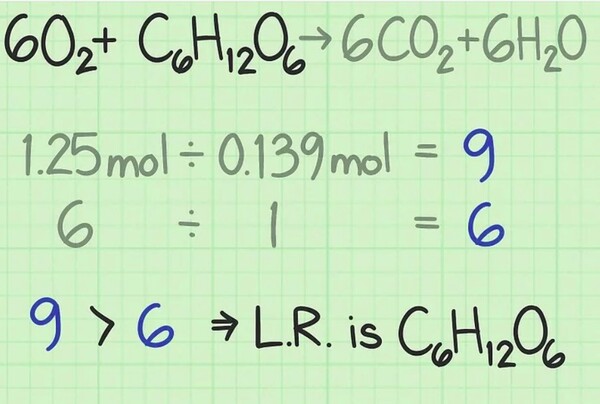
In maximum chemical reactions, one of the reactants can be used up earlier than the others. The one that gets used up first is known as the restricting reactant. This restricting reactant determines in this line how long the chemical response can take in a region and the theoretical yield you could expect. Compare the two ratios you calculated to become aware of the restricting reactant: [6]
In this example, you're starting with nine instances of as much oxygen as glucose, measured through a range of moles. The components tell you that your perfect ratio is six times as much oxygen as glucose. Therefore, you've got more oxygen than is required. Thus, the opposite reactant, glucose, in this case, is the restricting reactant.
Knowing the theoretical yield of your investments allows you to compare what you could have with your current holdings. Not sure how to calculate it? Here's a step-by-step guide.
- Choose your assets, such as gold or stocks.
- Find out how much the asset was worth on December 31st, considered one year in finance terms.
- Multiply this amount by 366 (days in a year) and divide that number by 100.
Investing in a piece of property that has high potential and yields good returns can be rewarding. With this in mind, you must know how to calculate theoretical yield. It would help if you had a formula of P x R x F, where P represents the property's present value, R is the interest rate, and F is the investment period. If you have enough cash, use your own money for this calculation.
In this example, the molar mass of CO2 is forty-four g/mol. (Carbon's molar mass is 12 g/mol and oxygen's is sixteen g/mol, so the total is 12 + 16 + 16 = forty-four.) Multiply 0.834 moles CO2 x forty-four g/mol CO2 = 36.7 grams. The theoretical yield of the test is 36.7 grams of CO2.
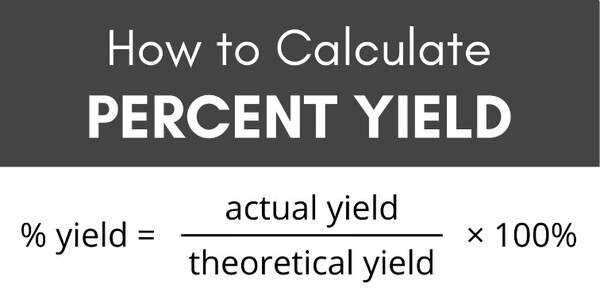
Theoretical yield formula. In a chemical response, the quantity of product shaped is decided by the amount of proscribing reactant used. Stoichiometry is used to expect this quantity of product. It is called the theoretical yield.
1. Balance the Reaction.
2. Identify the restricting reagent, that's the reagent with the fewest moles.
3. Divide the fewest variety of reagent moles through the stoichiometry of the product.
4. Multiply Step Three's result by the preferred product's molecular weight.
The fight began much sooner than she anticipated after catching and returning the flamingo.
In the ever-evolving landscape of dental care, making informed choices about dental implants is crucial for long-term success.
Discover the unique journey of 'Navigating Life's Beauty: A Cute Girl with Bad Eyesight' - a captivating tale of resilience, redefined style, and the triumph of character where imperfections shine brilliantly.
If you want to withdraw your Coinbase account to your PayPal account, you've come to the right place. Follow these simple steps, and you'll have your money quickly.


